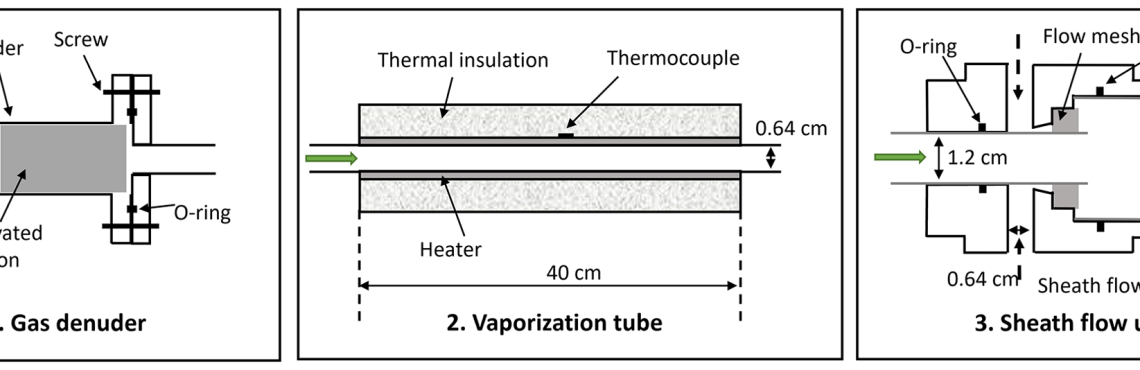
Characterization of the Vaporization Inlet for Aerosols (VIA) for online measurements of particulate highly oxygenated organic molecules (HOMs)
Jian Zhao, Valter Mickwitz, Yuanyuan Luo1, Ella Häkkinen, Frans Graeffe1, Jiangyi Zhang, Hilkka Timonen, Manjula Canagaratna, Jordan E. Krechmer, Qi Zhang, Markku Kulmala, Juha Kangasluoma, Douglas Worsnop, and Mikael Ehn
Atmospheric Measurements Techniques
Atmos. Meas. Tech., 17, 1527–1543, 2024
Publication Date: March 12, 2024
https://doi.org/10.5194/amt-17-1527-2024
© Author(s) 2024. This work is distributed under
the Creative Commons Attribution 4.0 License.
Abstract. Particulate matter has major climate and health impacts, and it is therefore of utmost importance to be able to measure the composition of these particles to gain insights into their sources and characteristics. Many methods, both offline and online, have been employed over the years to achieve this goal. One of the most recent developments is the Vaporization Inlet for Aerosols (VIA) coupled to a nitrate Chemical Ionization Mass Spectrometer (NO3-CIMS), but a thorough understanding of the VIA–NO3-CIMS system remains incomplete. In this work, we ran a series of tests to assess the impacts from different systems and sampling parameters on the detection efficiency of highly oxygenated organic molecules (HOMs) in the VIA–NO3-CIMS system. Firstly, we found that the current VIA system (which includes an activated carbon denuder and a vaporization tube) efficiently transmits particles (> 90 % for particles larger than 50 nm) while also removing gaseous compounds (> 97% for tested volatile organic compounds – VOCs). One of the main differences between the VIA and traditional thermal desorption (TD) techniques is the very short residence time in the heating region, on the order of 0.1 s. We found that this short residence time, and the corresponding short contact with heated surfaces, is likely one of the main reasons why relatively reactive or weakly bound peroxides, for example, were observable using the VIA. However, the VIA also requires much higher temperatures in order to fully evaporate the aerosol components. For example, the evaporation temperature of ammonium sulfate particles using the VIA was found to be about 100–150 °C higher than in typical TD systems. We also observed that the evaporation of particles with larger sizes occurred at slightly higher temperatures compared to smaller particles. Another major aspect that we investigated was the gas-phase wall losses of evaporated molecules. With a more optimized interface between the VIA and the NO3-CIMS, we were able to greatly decrease wall losses and thus improve the sensitivity compared to our earlier VIA work. This interface included a dedicated sheath flow unit to cool the heated sample and provide the NO3-CIMS with the needed high flow (10 L min−1). Our results indicate that most organic molecules observable by the NO3-CIMS can evaporate and be transported efficiently in the VIA system, but upon contact with the hot walls of the VIA, the molecules are instantaneously lost. This loss potentially leads to fragmentation products that are not observable by the NO3-CIMS. Thermograms, obtained by scanning the VIA temperature, were found to be very valuable for both quantification purposes and for estimating the volatility of the evaporating compounds. We developed a simple one-dimensional model to account for the evaporation of particles and the temperature-dependent wall losses of the evaporated molecules, and we thereby estimate the concentration of HOMs in secondary organic aerosol (SOA) particles. Overall, our results provide much-needed insights into the key processes underlying the VIA–NO3-CIMS method. Although there are still some limitations that could be addressed through hardware improvements, the VIA–NO3-CIMS system is a very promising and useful system for fast online measurements of HOMs in the particle phase.
